Lake Natron in Tanzania is one of the most tranquil lakes in Africa, but it’s also the source of some of the most eerie photographs ever taken – images that appear to show living animals turned to stone.
About Natron Lake
“Natron is typically a warm 80 degrees Fahrenheit and blood-red from bacteria, the only living organisms capable of surviving its lethal alkalinity.” It’s recently gained a reputation for washing up on its shores the bodies of small creatures, each wrapped in a lovely crusty shroud.
More than 2 million lesser flamingos (Phoenicopterus minor) use the small lake as their principal breeding habitat in Africa during the breeding season. The nests of the flamingos are established on little islands that grow in the lake during the dry season.
Lake Natron may be one of two alkaline lakes in East Africa, other one being Lake Bahi. Both are terminal lakes with no river or sea exit; they are supplied by hot springs and small rivers. Because they are shallow lakes in a hot climate, their water temperatures can exceed 106 degrees Fahrenheit (41 degrees Celsius) (41 degrees Celsius).
A proposed hydroelectric power plant on the Ewaso Ngiro River, the main river feeding Lake Natron, threatens the lake’s serenity and flamingo population. Because the lake is so remote (it wasn’t even found by Europeans until 1954), there are no safeguards in place to preserve it or its endangered flamingo population.

Brandt was struck by the stunningly well-preserved bodies of bats, flamingos, eagles, and swallows, and he documented the odd event with a series of images.
Substance Contained in Natron Lake
Lake Natron’s alkaline water has a pH as high as 10.5 and is so caustic that it can burn the skin and eyes of creatures who aren’t acclimated to it. The alkalinity of the water is caused by sodium carbonate and other minerals that pour into the lake from the neighbouring hills. And deposits of sodium carbonate, which was originally utilised in Egyptian mummification, work as a magnificent type of preservation for creatures unfortunate enough to die in Lake Natron’s waters.
The ancient Egyptians employed natron, which is a naturally occurring mixture of sodium carbonate decahydrate (Na2CO310H2O, a type of soda ash) and around 17 percent sodium bicarbonate (commonly known as baking soda, NaHCO3), as well as trace amounts of sodium chloride and sodium sulphate.
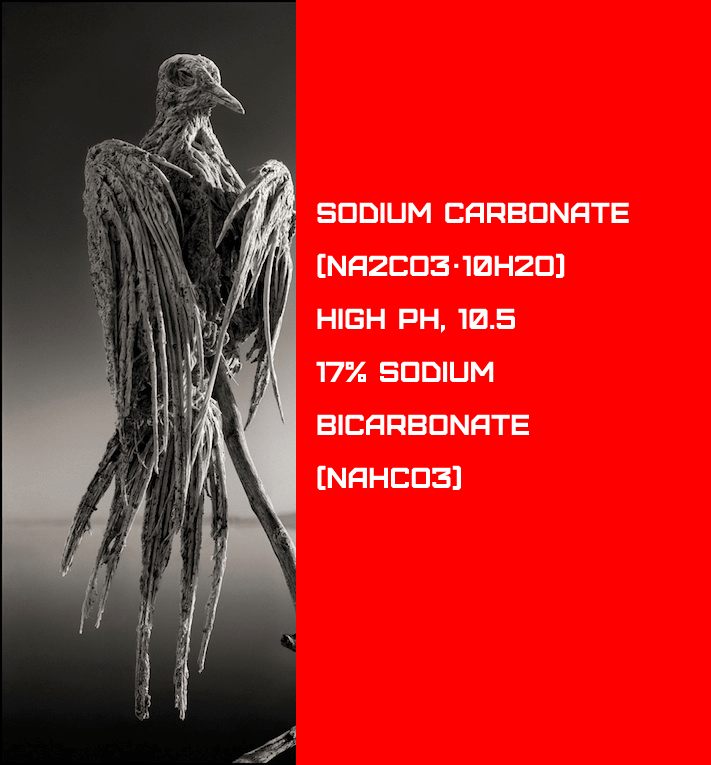
The ancient Egyptians utilised natron to preserve mummies, and when an animal dies in this water, its body is preserved by the natron, and these animals resemble statues, giving rise to the myth that they turned to stone.
Their bodies do not turn to stone, but they do become calcified.
Media Reports
Contrary to popular belief, the animal did not turn to stone and die after coming into contact with the lake’s water. Lake Natron’s alkaline waters, in reality, sustain a healthy ecology of salt marshes, freshwater wetlands, flamingos and other wetland birds, tilapia, and the algae that enormous flocks of flamingos feed on. In a book titled “Across the Ravaged Land,” photographer Nick Brandt has collected heartbreaking photographs of the lake and its corpses.
Brandt discovered the bones of flamingos and other animals with sharply defined chalky sodium carbonate deposits outlining their bodies. “I unexpectedly found the creatures — all kinds of birds and bats — washed up along Lake Natron’s shoreline,” Brandt writes in his book. “No one knows for sure how they die, however… the water has an exceptionally high soda and salt concentration, so high that it would remove the ink from my Kodak film boxes in a matter of seconds.”

“I took these creatures as I found them on the shoreline, and then arranged them in ‘living’ postures, bringing them back to ‘life,’ as it were,” Brandt wrote, alluding to how the animals were relocated. “Resurrected, alive in death.”
What Happen If We Jump Into Natron?
It wouldn’t kill you right away. The water can be warm (though not dangerous), yet it has a pH of 10.5 and is strongly basic. Alkalinity differs slightly: Alkalinity is a measure of water’s buffering capacity, or its ability to withstand abrupt changes in pH, whereas pH is a measure of how acidic or basic the water is.
In either situation, you’d quickly find yourself in uncomfortable water. When you immerse your hand in very alkaline water, you will begin to feel greasy as part of the fat beneath the skin dissolves.
If you got any of the liquid into your eyes and on your skin , you would start getting devoured by the liquid(it wouldn’t pain as suggested by a commentator) in numerous regions of your body. The liquid is beginning to “burn” you, not with fire but devour you. This may be connected to the carcasses of animals discovered around and on the lake.
(You will be burned since your skin and eyes are unaccustomed to the extreme alkalinity and warmth of the water.)
You are going to be burned due to the obvious high temperature throughout the lake which is roughly 140 F. It’s rather hot.
There wouldn’t be no pain, certainly there would be a pain as your skin starts being exposed to air. If you can endure the lake bucking your skin away, sure, but it doesn’t care if you have a high pain tolerance; it can dehydrate you due to reverse osmosis, and you will be drained dry by the high salt content in the water.